Understanding the NO3 Lewis structure is essential for anyone diving into the field of chemistry. The nitrate ion (NO3-) plays a crucial role in various chemical reactions and applications, from fertilizers to environmental studies. By demystifying this structure, we gain insight into the chemical behavior of nitrates and their significance beyond the lab—such as their impact on stock markets and environmental legislation. Dive into the details of the NO3 Lewis structure, and discover its connections to real-world issues like the cdk cyber attack and contemporary cultural trends.
Understanding the NO3 Lewis Structure: Key Concepts You Need to Know
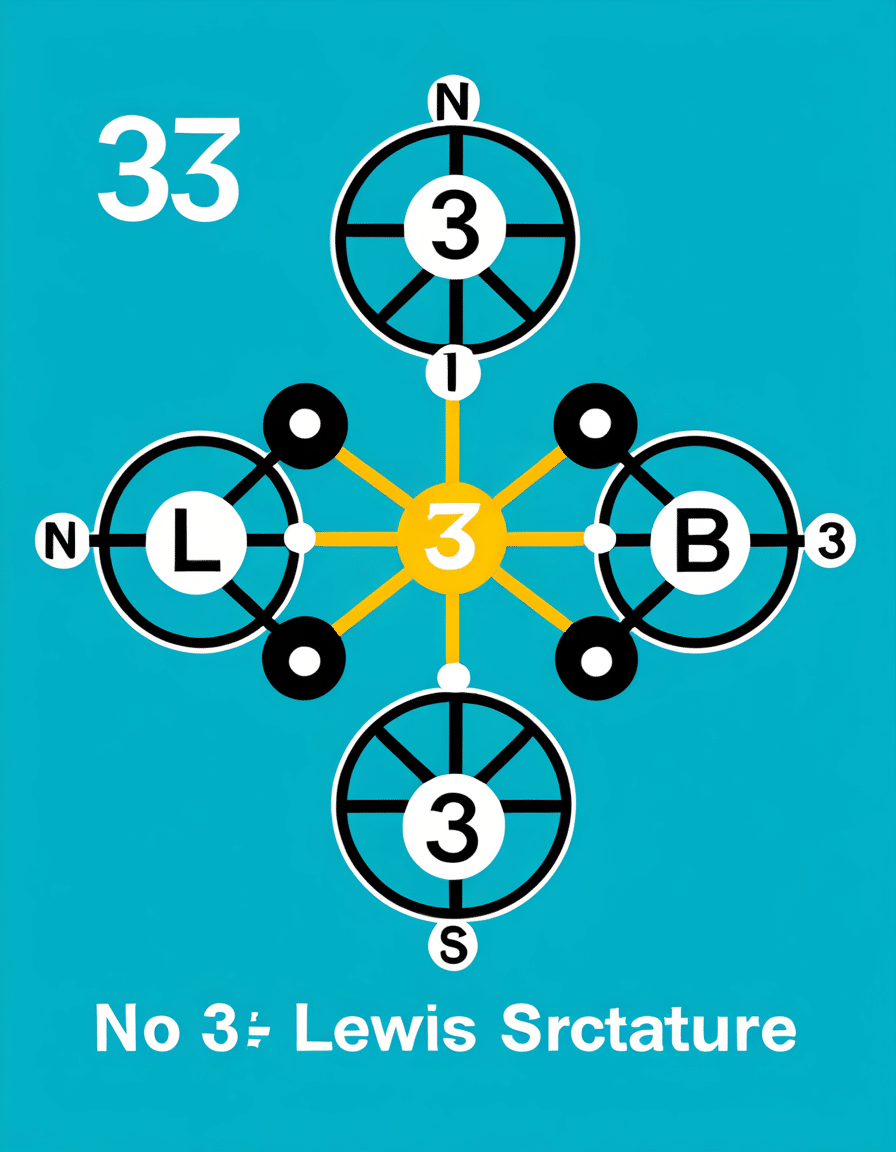
The nitrate ion (NO3-) consists of one nitrogen atom covalently bonded to three oxygen atoms. The nitrogen atom acts as the central player in this molecular configuration due to its lower electronegativity compared to oxygen. In the classic depiction of the NO3 Lewis structure, you’ll find one nitrogen forming a double bond with one oxygen atom while forming single bonds with the remaining two. This arrangement leads to resonance structures, a key feature that enhances the ion’s stability.
When sketching the NO3 Lewis structure, understanding the arrangement of these atoms is paramount. Not only does it dictate the ion’s chemical properties, but it also sets the stage for discussions about its environmental relevance. High nitrate levels in water sources reflect runoffs from agricultural practices—a phenomenon linking chemistry to environmental science and regulatory policies aimed at addressing these pollution concerns.
Top 4 Essential Aspects of the NO3 Lewis Structure
The nitrate ion’s structure—central nitrogen bonded to three oxygens—forms the backbone of many reactions. The nitrogen’s connection via two single bonds and one double bond creates a stable configuration, but hang on, there’s more! This setup allows for resonance, meaning that the position of the double bond can shift among the oxygen atoms. This fluidity offers the NO3 ion versatility, crucial for its reactions with compounds in soil and water.
When analyzing the NO3 Lewis structure, remember that resonance is key. It’s not just one static picture; it’s several forms that contribute to the ion’s overall stability. Each resonance structure represents a different way the bonds and charges can be arranged, which in turn, leads to a lowered energy state of the ion. The nitrate ion achieves its overall -1 charge through this electron delocalization, showcasing nature’s knack for efficiency.
Let’s talk geometry! The NO3 ion boasts a trigonal planar shape, which stems from the sp2 hybridization of the nitrogen atom. The bond angles sit at about 120 degrees—perfect for maximizing interaction with other molecules. This shape not only aids in its role in agricultural fertilizers but also influences how it interacts during acidic reactions forming nitric acid (HNO3).
Understanding the NO3 Lewis structure opens doors to discussions on real-world implications. Take groundwater pollution, for instance. Nitrates in runoff can lead to algal blooms—an environmental nightmare that limits oxygen levels in water and harms aquatic life. Strikingly similar is the way that cyber threats, like the cdk cyber attack, push industries like tech and agriculture to adapt not only chemically but also digitally. Protecting data and chemical safety requires a well-rounded strategy.
The ramifications of nitrate pollution touch on public health and regulatory discussions that resonate widely. Companies tied to fertilizer use, such as those in agriculture and even the industries relying on applications for growth, must navigate the fine line between chemical usage and environmental protection. With public scrutiny on the rise, corporations must prepare for shifts in legislation impacting their future.
Real-World Impacts: NO3 and Beyond
The significance of the NO3 ion doesn’t just reside within textbooks; it branches out into economic and cultural realms as well. Take Lyft, for instance. Their stock performance can swing due to regulations involving nitrates and other nitrogen compounds—representing a direct link between environmental policy and corporate financial health. As markets react to changes in legislation, savvy investors watch these trends closely, knowing that fluctuations can mean profit or loss.
Additionally, within cultural dynamics, we see how sustainability plays a role in various industries, including fashion. Brands that adopt sustainable practices, much like those re-evaluating kimono styles for eco-friendliness, echo the sentiments around chemical use. The merging of tradition with modernity reflects an ongoing dialogue about corporate responsibility, making these narratives compelling for consumers who prioritize the environment.
Innovative Perspectives on Chemical Structures and Their Learnings
Exploring the NO3 Lewis structure exemplifies how chemistry intersects with broader societal matters. Contemplating the implications around nitrate usage brings attention to environmental sustainability, corporate governance, and cultural influences. These reflections urge us to realize that engaging with chemical structures enriches discussions far beyond the laboratory.
In an age where chemistry meets economics and culture, even something as small as a nitrate ion can create ripples throughout industries. From agricultural practices affecting stock performance, like those for Pinterest stock, to environmental policies that influence corporate strategies, it’s vital for sectors to reflect on responsibilities and rethink innovative practices. The dialogue surrounding the popular vote in 2025 versus 2020 further illustrates how interconnected our decisions have become.
By investigating the layers of the NO3 Lewis structure, we grasp how crucial these elements are in shaping our environmental, economic, and social landscape. It’s through this exploration that we harness the power of knowledge to foster innovative solutions, urging all sectors to forge a brighter, more sustainable future for the world we inhabit.
Fun Trivia and Interesting Facts about the NO3 Lewis Structure
The Basics of NO3 Lewis Structure
Did you know that the nitrate ion, represented as NO3, is a key player in both environmental science and agriculture? Its Lewis structure reveals a central nitrogen atom bonded to three oxygen atoms, showcasing how electrons are shared to create stability in molecules—as if they’re the cast of “The Godfather,” each playing a vital role in a well-orchestrated performance. Fascinatingly, this ion is commonly found in fertilizers, illustrating its significance in boosting plant growth while also reminding us of the delicate balance in ecosystems, similar to understanding Patrick Mahomes’s impressive salary in the NFL, which reflects not only talent but also strategy in navigating competitive sports.
Bonding Arrangements and Their Impact
In the NO3 Lewis structure, one of the oxygen atoms is double-bonded to nitrogen while the others have single bonds, with one carrying a negative charge. This arrangement helps explain the molecular ion’s resonance, where the double bond can shift between the oxygen atoms, much like how the popularity of items like box wine can fluctuate according to trends and consumer preferences. If you’ve ever enjoyed a good debate about the dynamics of the popular vote in elections, like comparing the popular vote in 2020 and 2025, you can appreciate how small changes can lead to significant outcomes, just like the alternating double bond in our nitrate ion.
Fun Connections to Everyday Life
The diversity of NO3’s applications extends beyond chemistry; it even pops up in topics like sports gear. Take, for example, the Kyrie 8 basketball shoes, which are engineered for performance—much like the precise bonds in the NO3 Lewis structure help molecules achieve stability. And speaking of balancing acts, did you know that Angelina Jolie’s children have been in the spotlight just as much as some famous film casts? Their lives are a reminder of how various components come together to create a beautifully complicated picture, reflecting the layered structure of the nitrate ion in chemical reactions.
Understanding the intricacies of NO3 can enhance our grasp of numerous systems in nature, paralleling how we appreciate the nuances in a sitcom like Yes Dear. There’s always something new to learn, so jump right into the exciting world of chemical bonding and see how even the smallest ions can have a big impact!